50+ pages how to identify an unknown metal using calorimetry 3.4mb. This allows substances to be identified using their specific heat. We will be using density and specific heat also known as heat capacity or specific heat capacity. When attempting to identify a sample of an unknown metal the first thing you should always do is hold a magnet up to it. Check also: unknown and understand more manual guide in how to identify an unknown metal using calorimetry The purpose of the lab calculates the specific heat value of a known and the unknown metal through the observations and.
Because calorimetry is used to measure the heat of a reaction it is a crucial part of thermodynamics. Also using the heat capacity of water one can figure out the heat capacity of an unknown substance by putting it in water and measure the temperature change of the water and the unknown substance.

Alkali Metals And Alkaline Earth Metals A Printable From Help Teaching Alkaline Earth Metals Alkali Metal Help Teaching
| Title: Alkali Metals And Alkaline Earth Metals A Printable From Help Teaching Alkaline Earth Metals Alkali Metal Help Teaching |
| Format: PDF |
| Number of Pages: 238 pages How To Identify An Unknown Metal Using Calorimetry |
| Publication Date: November 2019 |
| File Size: 2.6mb |
| Read Alkali Metals And Alkaline Earth Metals A Printable From Help Teaching Alkaline Earth Metals Alkali Metal Help Teaching |
 |
In this lab we will be using lab techniques and basic chemical concepts to identify an unknown metal.

Unk2 421 g 435 g 1006 g. Knowing the heat capacity of water it is possible to find how well its environment insulates it. Temperature was recorded when water reached a boil. Unk1 825 g 435 g 100 g. Identification of an Unknown Metal In this lab we will be using lab techniques and basic chemical concepts to identify an unknown metal. Every metal has a unique set of properties.
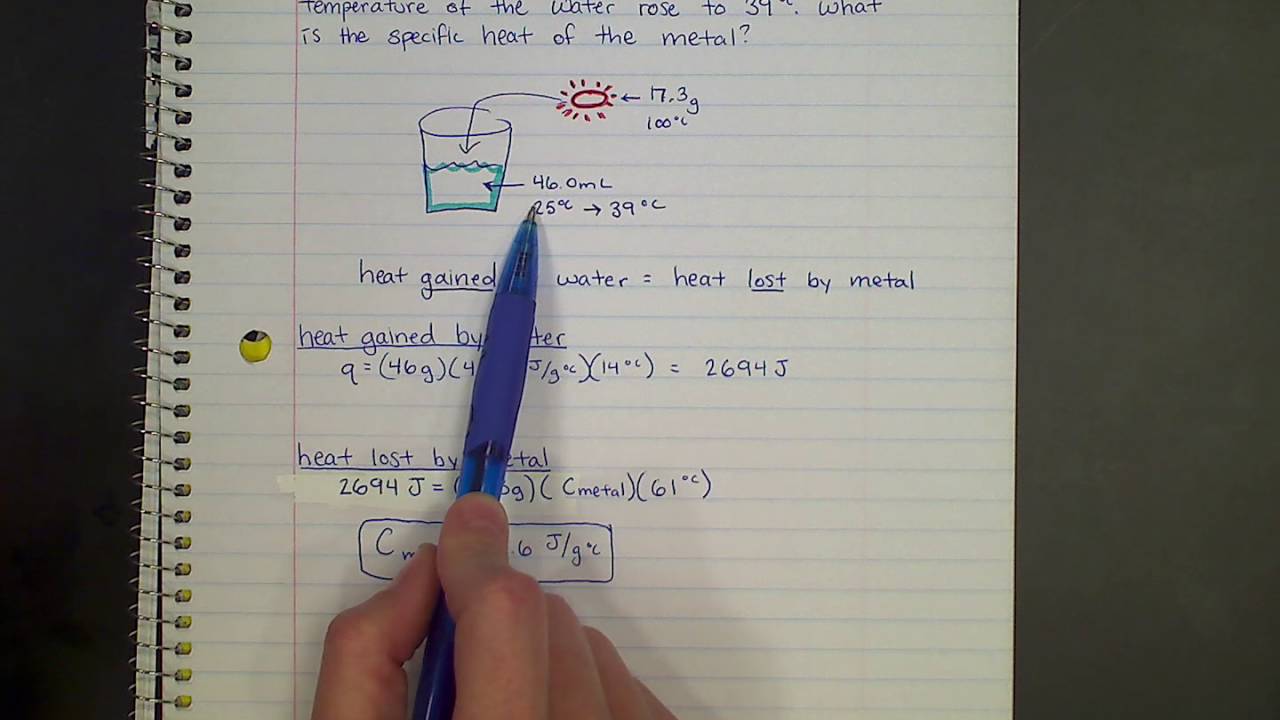
Calorimetry Unknown Metals
| Title: Calorimetry Unknown Metals |
| Format: PDF |
| Number of Pages: 334 pages How To Identify An Unknown Metal Using Calorimetry |
| Publication Date: February 2020 |
| File Size: 6mb |
| Read Calorimetry Unknown Metals |
 |

Introduction Matter Is Posed Of Molecules That Are In Constant Motion When These Molecules Gain Enough Ener Chemistry Labs Chemistry Classroom Chemistry
| Title: Introduction Matter Is Posed Of Molecules That Are In Constant Motion When These Molecules Gain Enough Ener Chemistry Labs Chemistry Classroom Chemistry |
| Format: PDF |
| Number of Pages: 129 pages How To Identify An Unknown Metal Using Calorimetry |
| Publication Date: December 2020 |
| File Size: 1.2mb |
| Read Introduction Matter Is Posed Of Molecules That Are In Constant Motion When These Molecules Gain Enough Ener Chemistry Labs Chemistry Classroom Chemistry |
 |

In This Experiment We Will Use A Styrofoam Cup As Our Calorimeter Ice Will Be Placed Directly Into A Meas Chemistry Labs Teaching Chemistry Chemistry Classroom
| Title: In This Experiment We Will Use A Styrofoam Cup As Our Calorimeter Ice Will Be Placed Directly Into A Meas Chemistry Labs Teaching Chemistry Chemistry Classroom |
| Format: ePub Book |
| Number of Pages: 313 pages How To Identify An Unknown Metal Using Calorimetry |
| Publication Date: March 2018 |
| File Size: 1.5mb |
| Read In This Experiment We Will Use A Styrofoam Cup As Our Calorimeter Ice Will Be Placed Directly Into A Meas Chemistry Labs Teaching Chemistry Chemistry Classroom |
 |

An Unknown Metal Of Mass 192 G Heated To A Temperature Of 100 C Was Immersed Into A Brass
| Title: An Unknown Metal Of Mass 192 G Heated To A Temperature Of 100 C Was Immersed Into A Brass |
| Format: ePub Book |
| Number of Pages: 238 pages How To Identify An Unknown Metal Using Calorimetry |
| Publication Date: August 2019 |
| File Size: 810kb |
| Read An Unknown Metal Of Mass 192 G Heated To A Temperature Of 100 C Was Immersed Into A Brass |
 |

Irydium Virtual Online Chemistry Lab Chemistry Labs Teaching Chemistry Chemistry Education
| Title: Irydium Virtual Online Chemistry Lab Chemistry Labs Teaching Chemistry Chemistry Education |
| Format: PDF |
| Number of Pages: 259 pages How To Identify An Unknown Metal Using Calorimetry |
| Publication Date: April 2021 |
| File Size: 1.8mb |
| Read Irydium Virtual Online Chemistry Lab Chemistry Labs Teaching Chemistry Chemistry Education |
 |

Chemistry Lab Specific Heat Of A Metal Chemistry Labs Chemistry Science Student
| Title: Chemistry Lab Specific Heat Of A Metal Chemistry Labs Chemistry Science Student |
| Format: PDF |
| Number of Pages: 184 pages How To Identify An Unknown Metal Using Calorimetry |
| Publication Date: August 2020 |
| File Size: 5mb |
| Read Chemistry Lab Specific Heat Of A Metal Chemistry Labs Chemistry Science Student |
 |

Electron Configuration Chemwiki Electron Configuration Electrons Chemistry
| Title: Electron Configuration Chemwiki Electron Configuration Electrons Chemistry |
| Format: PDF |
| Number of Pages: 325 pages How To Identify An Unknown Metal Using Calorimetry |
| Publication Date: June 2020 |
| File Size: 2.1mb |
| Read Electron Configuration Chemwiki Electron Configuration Electrons Chemistry |
 |

Chemistry Lab Specific Heat Of A Metal Chemistry Labs Science Chemistry Teaching Chemistry
| Title: Chemistry Lab Specific Heat Of A Metal Chemistry Labs Science Chemistry Teaching Chemistry |
| Format: eBook |
| Number of Pages: 331 pages How To Identify An Unknown Metal Using Calorimetry |
| Publication Date: January 2019 |
| File Size: 2.3mb |
| Read Chemistry Lab Specific Heat Of A Metal Chemistry Labs Science Chemistry Teaching Chemistry |
 |

Experiment Specific Heat Capacity Of Water Experiments Digital Learning Edison Light Bulbs
| Title: Experiment Specific Heat Capacity Of Water Experiments Digital Learning Edison Light Bulbs |
| Format: eBook |
| Number of Pages: 333 pages How To Identify An Unknown Metal Using Calorimetry |
| Publication Date: August 2021 |
| File Size: 1.3mb |
| Read Experiment Specific Heat Capacity Of Water Experiments Digital Learning Edison Light Bulbs |
 |

On Awesome Chemistry Resources
| Title: On Awesome Chemistry Resources |
| Format: eBook |
| Number of Pages: 162 pages How To Identify An Unknown Metal Using Calorimetry |
| Publication Date: May 2017 |
| File Size: 1.6mb |
| Read On Awesome Chemistry Resources |
 |

Calculating The Specific Heat Of A Hot Piece Of Metal Dropped Into Water
| Title: Calculating The Specific Heat Of A Hot Piece Of Metal Dropped Into Water |
| Format: eBook |
| Number of Pages: 348 pages How To Identify An Unknown Metal Using Calorimetry |
| Publication Date: August 2019 |
| File Size: 1.35mb |
| Read Calculating The Specific Heat Of A Hot Piece Of Metal Dropped Into Water |
 |
Unk3 245 919 255. Temperature was recorded when water reached a boil. Because calorimetry is used to measure the heat of a reaction it is a crucial part of thermodynamics.
Here is all you have to to learn about how to identify an unknown metal using calorimetry How to Calculate the Specific Heat Capacity of an Unknown Metal through Calorimetry - YouTube. We will be using density and specific heat also known as heat capacity or specific heat capacity. Calorimeter C Initial metal temperature C Final temperature C Cu 209 963 229. Electron configuration chemwiki electron configuration electrons chemistry experiment specific heat capacity of water experiments digital learning edison light bulbs calculating the specific heat of a hot piece of metal dropped into water introduction matter is posed of molecules that are in constant motion when these molecules gain enough ener chemistry labs chemistry classroom chemistry alkali metals and alkaline earth metals a printable from help teaching alkaline earth metals alkali metal help teaching an unknown metal of mass 192 g heated to a temperature of 100 c was immersed into a brass The unknown metal is Copper Materials.